
 |
|
|||||||
| Image of the Day Images that will blow your mind - every day. [Blog] [RSS] [XML] |
 |
|
|
Thread Tools | Rate Thread | Display Modes |
|
|
#1 |
|
Radical Centrist
Join Date: Jan 2001
Location: Cottage of Prussia
Posts: 31,423
|
9/27: Lake Michigan gets lighter
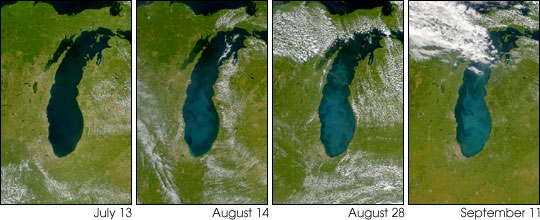 From the earth science pic of the day: The above series of visible satellite images shows changes in the appearance of Lake Michigan over the course of several weeks. The images are from the Sea-viewing Wide Field-of-view Sensor (SeaWiFS) aboard the Orbview-2 satellite. The bright color that appears in late summer is probably caused by calcium carbonate chalk in the water. Lake Michigan always has an abundance of calcium carbonate because the floor of the lake is composed largely of limestone. During most of the year, this calcium carbonate remains dissolved in the cold water, but at the end of summer the lake water warms up, thus lowering the solubility of the calcium carbonate. As a result, it precipitates out of the water, forming clouds of very small solid particles. From space these particles appear as bright swirls. The phenomenon is referred to as a whiting event. A similar event occurred in 1999. It's also possible that a bloom of the algae Microcystis is responsible for the color change, but unlikely because of Lake Michigan's depth and size. |
|
|

|
|
|
#2 |
|
Master of the Domain
Join Date: Sep 2001
Location: AZ
Posts: 221
|
wait a second
I thought the warmer water is, the faster it will dissolve something? And that warm water can hold more dissolved stuff than cold water?
|
|
|

|
|
|
#3 | |
|
Guest
Posts: n/a
|
Re: wait a second
Quote:
It says though that the solubility of the calcium carbonate is lowered, so therefore it isn't dissolved as easily, and I guess therefore clouds up the water... |
|

|
 |
| Currently Active Users Viewing This Thread: 1 (0 members and 1 guests) | |
|
|